Contents
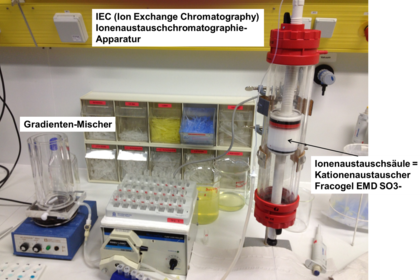
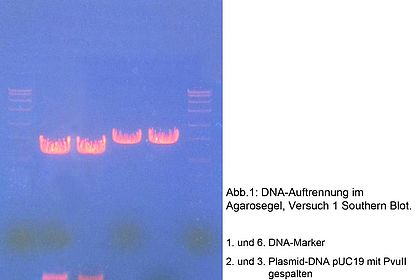
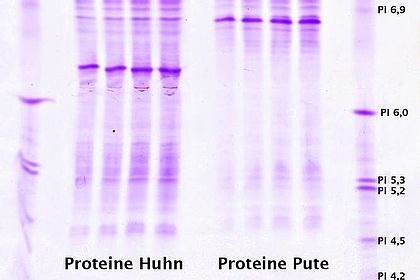
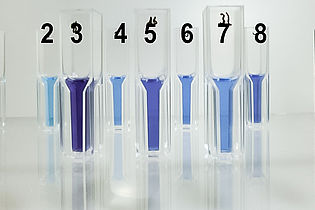
Chromatography is a key method used in biotechnology processing to isolate proteins from culture broths for example and purify them. Students learn about different chromatographic techniques and how to operate the Äkta system. Experiments: purification of lactoperoxidase from unpasteurised milk and recombinant GFP from Escherichia coli
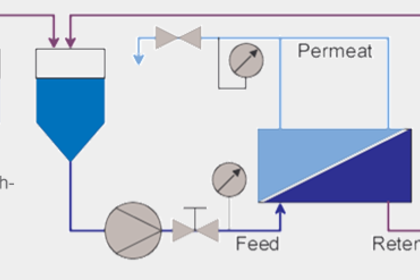
Ultrafiltration brings about the separation of solutions made up of constituents with high and low molecular weights. It is state-of-the-art in the production of fermentation products. Experiment: reconcentration of yeast cells and desalination of a haemoglobin solution
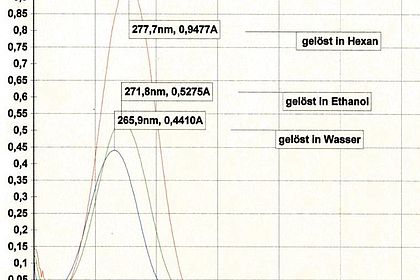
With liquid-liquid extraction, students become familiar with a further method to separate organic active substances from a culture broth. Experiment: extraction of BCG from an aqueous medium into the solvent ethyl acetate and back extraction
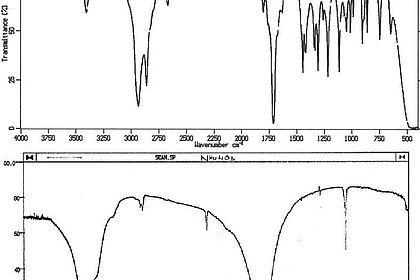
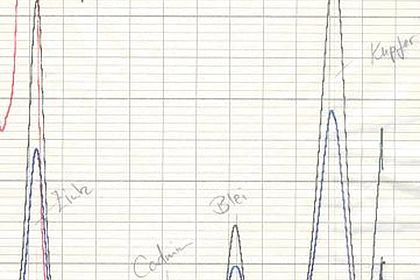
The most important operations in the processing of biotechnology products include the digestion of tissues and cells. Students learn about the variety of digestion and degradation techniques in a range of experiments. Experiments: various stations with digestion and degradation machines.
Head of Laboratory:
Prof.Dr. Cristina Sirrenberg-Cruciat
Prof.Dr. Andreas Scheibe
Assistant:
Dipl.-Ing.(FH) Bülent Turan
Laboratory: S04.107
Laboratory phone: -3537