Contents
How is the coating applied to the workpiece? Under which conditions do we achieve the desired optical and technological properties of the coating layer? How can we design our methods and processes so as to minimise the negative effects on the environment and decrease our operating costs at the same time?
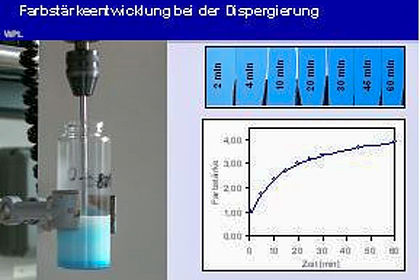
These are questions which are answered in the Application Technology Laboratory through the practical utilisation of different application methods. We get to know the most important methods of paint application and obtain a first impression of how we can decisively improve the quality of the paint layer by optimising the application parameters.
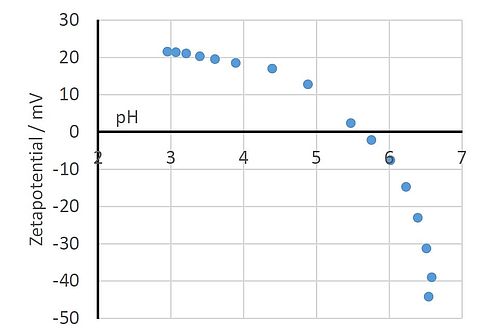
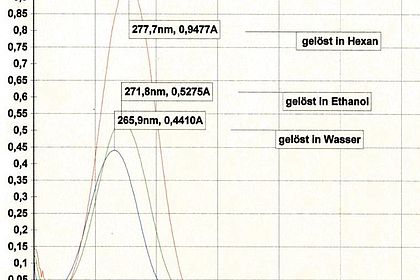
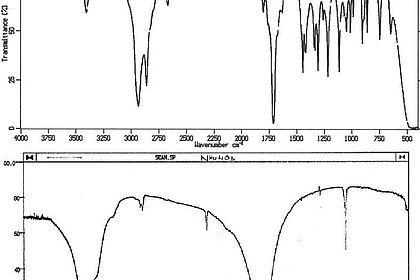
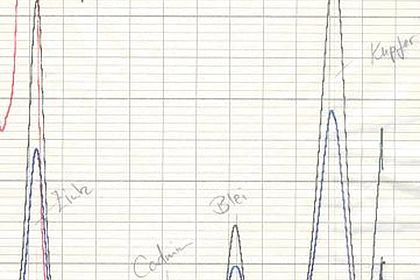
An important aspect here is the correct practical handling of the various measurement methods to assess the optical and technical properties of the paint layer. This includes hue, brilliance and roughness as well as hardness, scratch resistance or elasticity.
Application techniques such as dip and spray coating or electrostatic power coating are considered. Manual systems as well as automated equipment with robot support are used here. Some of the laboratory work also involves looking at the drying process.
Head of Laboratory: Prof. Dr.-Ing. Hendrik Dubbe
Assistant: Dipl.-Ing. (FH) Anja Stutz
Laboratory: S 10.208, S 10.210
Laboratory phone: -3517, -3518